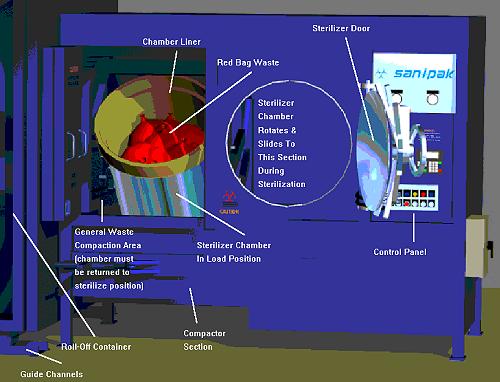
| 50mL tubes containing CI's. One is placed inside of a glove |
Data logger placed inside of a glove |
A second data logger is added |
The first gown is wrapped around the load |
A total of 5 gowns are wrapped sequentially |
 |
 |
 |
 |
 |
| The load is placed in a red bag |
The red bag is sealed |
The red bag is placed inside of the clear liner bag |
The clear bag is tied |
The load is placed in the chamber |
 |
 |
 |
 |
 |
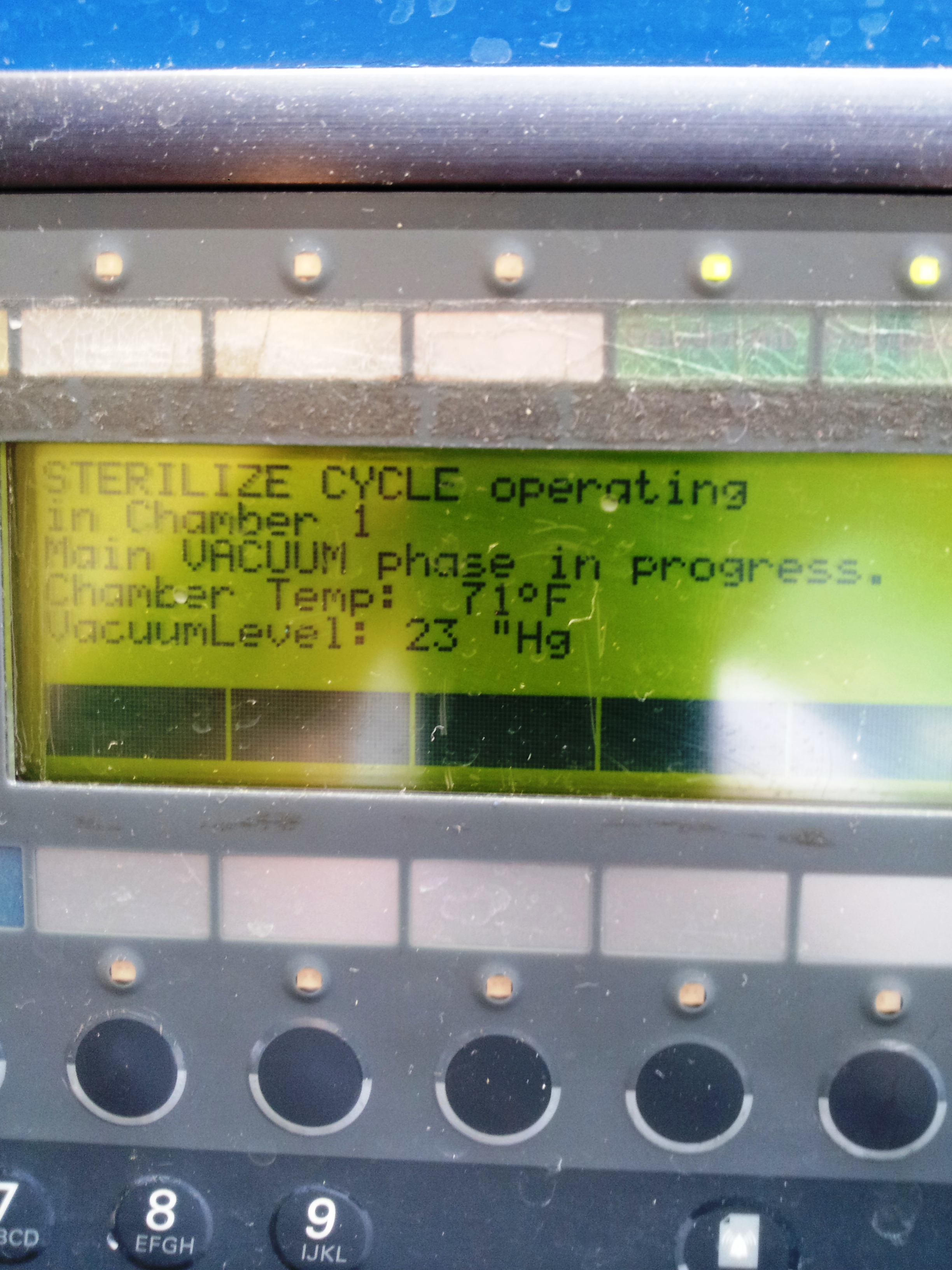
| The initial vacuum is drawn to 24"Hg |
Exposure phase is 40 minutes at 287oF |
Cycle complete |
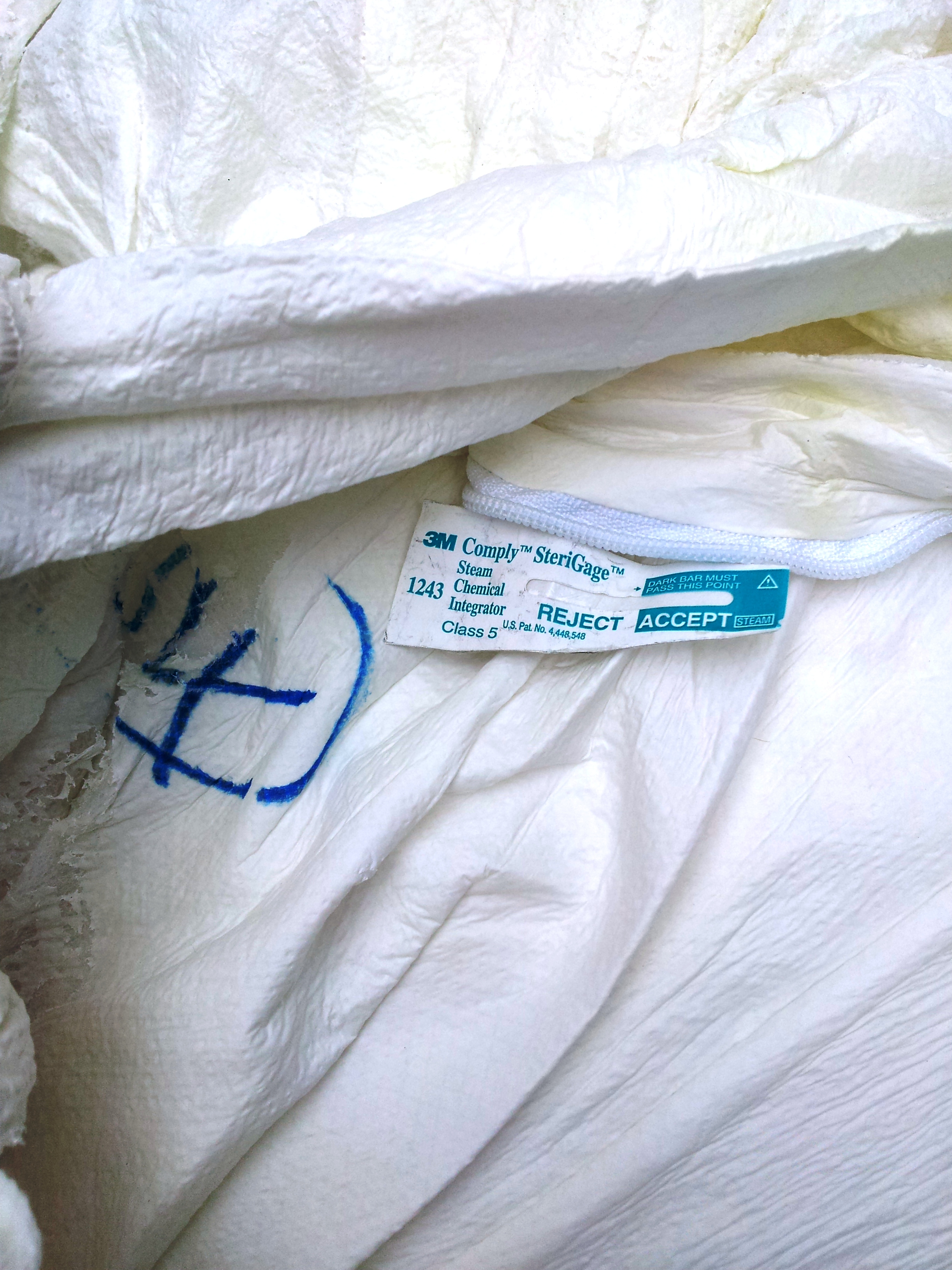
Red bag was breached. Clear bag was intact |
CI at gown layer 4 showed zero exposure |
 |
 |
 |
 |
 |